A range of energies that electrons can have in a solid. In a single atom, electrons exist in discrete energy levels. In a crystal, in which large numbers of atoms are held closely together in a lattice, electrons are influenced by a number of adjacent nuclei and the sharply defined levels of the atoms become bands of allowed energy (see illustration); this approach to energy levels in solids is often known as the band theory. Each band represents a large number of allowed quantum states. Between the bands are forbidden bands . The outermost electrons of the atoms (i.e. the ones responsible for chemical bonding) form the valence band of the solid. This is the band, of those occupied, that has the highest energy.
The band structure of solids accounts for their electrical properties. In order to move through the solid, the electrons have to change from one quantum state to another. This can only occur if there are empty quantum states with the same energy. In general, if the valence band is full, electrons cannot change to new quantum states in the same band. For conduction to occur, the electrons have to be in an unfilled band — the conduction band . Metals are good conductors either because the valence band and the conduction band are only half-filled or because the conduction band overlaps with the valence band; in either case vacant states are available. In insulators the conduction band and valence band are separated by a wide forbidden band and electrons do not have enough energy to ‘jump’ from one to the other.
In intrinsic semiconductors the forbidden gap is narrow and, at normal temperatures, electrons at the top of the valence band can move by thermal agitation into the conduction band (at absolute zero, a semiconductor would act as an insulator). Doped semiconductors have extra bands in the forbidden gap.
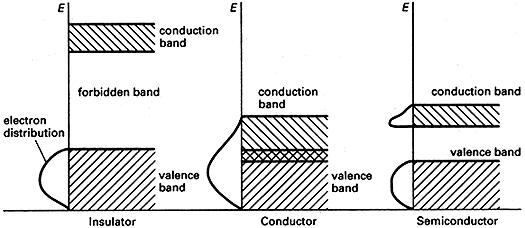
Energy bands
- Markoffian process
- martensite
- mascon
- maser
- mass
- mass action
- mass decrement
- mass defect
- mass number
- mass spectrum
- mass-energy equation
- matrix
- matrix mechanics
- maximum and minimum thermometer
- maximum permissible dose
- maxwell
- Maxwell's demon
- Maxwell's equations
- Maxwell, James Clerk
- Maxwell-Boltzmann distribution
- McLeod gauge
- mean
- mean free path
- mean free time
- mean life